Tokyo, Japan: A research breakthrough from Chiba University offers a major stride toward affordable large-scale hydrogen production from water.
Scientists have discovered that modifying platinum cathodes with naturally occurring purine bases – such as caffeine and purine – can increase the hydrogen evolution reaction (HER) activity by up to four times, significantly cutting platinum usage in electrolyzers.
Purine Modification Enhances HER for Cost-Effective Hydrogen Production from Water
Hydrogen production from water, though simple in principle, faces economic challenges due to the high cost of electrolyzers, priced at $2,000–$2,600 per kilowatt in 2024.
Also Read: H2 Carbon Zero Secures USD 850K Seed Funding from Venture Catalysts
To make green hydrogen viable, the U.S. Department of Energy targets cost reductions to $2/kg by 2025 and $1/kg by 2030. The latest findings from Chiba University researchers could help achieve these benchmarks by improving the efficiency of electrolysis and reducing platinum dependence.
The study, led by Dr Syunnosuke Tanaka and Professor Masashi Nakamura from the Graduate School of Engineering, Chiba University, found that adding purine bases – organic molecules found in DNA and RNA – can enhance HER activity by 4.2 times.
This advancement in hydrogen production from water reduces the quantity of platinum needed, lowering the overall system cost. Their research was published online on September 4, 2025, and in Volume 172 of the International Journal of Hydrogen Energy on September 26, 2025.
“Platinum scarcity limits the scalability of water electrolysis and fuel cell technologies. Reducing platinum loading is an essential step toward practical, cost-effective catalysts,” said Prof. Nakamura.
Molecular Insights into Enhanced Hydrogen Evolution Reaction
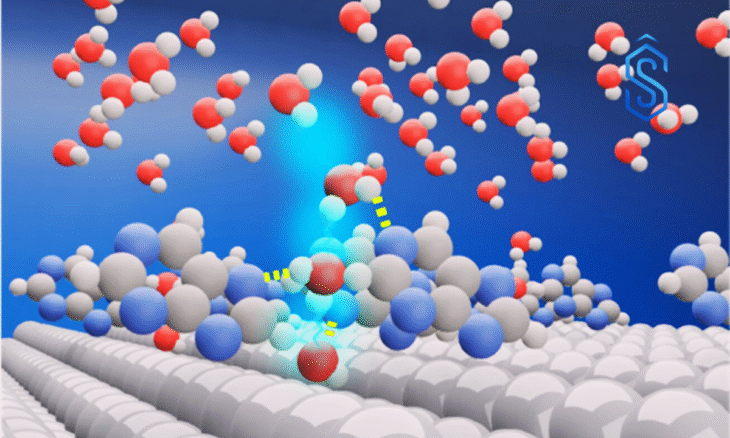
During electrolysis, hydrogen ions (H⁺) are adsorbed onto catalysts before forming hydrogen gas. In alkaline media, catalysts must first break the OH bond of water – a step that increases energy consumption. The team found that caffeine and other purine derivatives promote hydrogen adsorption, accelerating the reaction even under alkaline conditions.
Also Read: Chung-Ang University Breakthrough in Seawater Electrolysis with Chloride-Resistant Catalysts
Among the compounds tested – caffeine, xanthine, purine, theophylline, and theobromine—purine and theophylline delivered the strongest performance, improving HER activity by 4.2 and 5 times, respectively.
Structural analysis revealed that purine molecules create a cage-like hydrogen-bond network with surrounding water, facilitating OH⁻ removal and reducing the energy barrier for HER.
When applied to platinum/carbon catalysts, purine modification improved HER activity by 3.2 times in alkaline lithium hydroxide solutions. This approach offers a scalable and eco-friendly pathway to lower hydrogen production costs using naturally abundant materials.
“Water electrolysis using renewable energy is key to achieving carbon neutrality. Our catalyst innovation contributes to cost reduction and improved energy conversion efficiency in hydrogen production systems,” Prof. Nakamura added.
The discovery marks a crucial advancement toward sustainable and economically viable hydrogen production from water, aligning with global decarbonization goals.